Acumed Reaches Agreement Regarding Patent Infringement of Acutrak Screw
December 21, 2016
Acumed is pleased to announce that it has reached a resolution of a patent infringement dispute involving Acumed’s US Patent No. 6,030,162 and the Skeletal Dynamics REDUCT Headless Compression Screws (Acumed LLC v. Skeletal Dynamics LLC Case No. 3:15-CV-01581, filed in US District Court for the District of Oregon). As part of the settlement, Skeletal Dynamics has agreed to license Acumed’s patent for the remainder of its term.
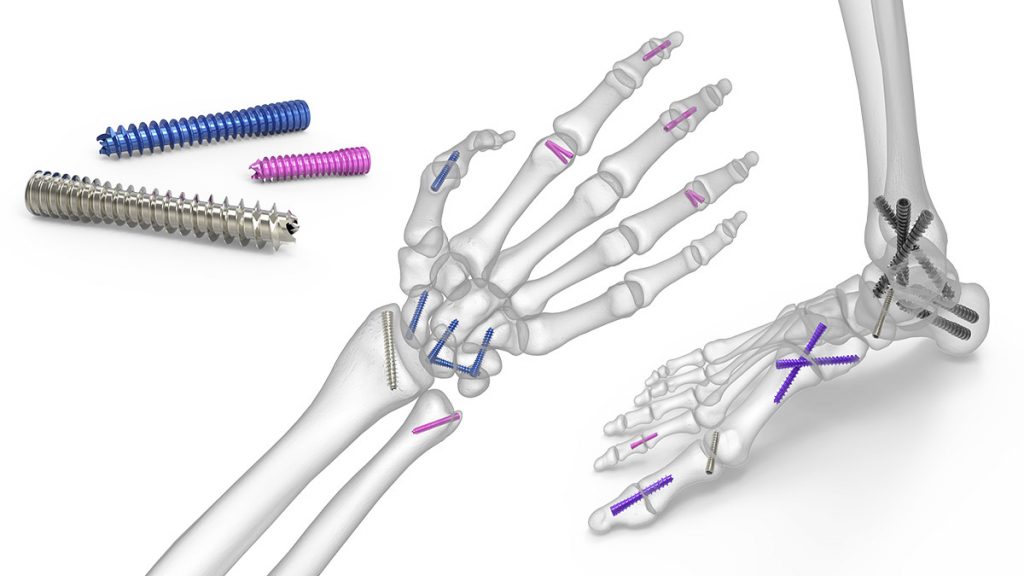
The patent pertains to the design of one of Acumed’s flagship products, the Acutrak® Headless Compression Screw. Introduced in 1994, Acutrak is the original fully threaded headless compression screw with continuously variable thread pitch.
The unique, continuously variable thread pitch ensures each screw rotation threads new bone along the Acutrak screw’s entire length. As each successive individual thread advances faster than the trailing thread counterpart, the conical shape becomes seated into bone. This radial expansion of the screw threads, combined with their axial advancement, creates the ability to reduce and compress bone fragments without a traditional screw head.
In this way, Acutrak technology overcame the limitations of conventional bone screws that could not pass threads across a fracture site to create compression. By allowing each thread along the entire length of the screw to aid in reduction and compression, compression can be maintained as the threads cross the fracture site.1
Traditional bone screws have a narrow window of compression. The Acutrak screw has a larger window of compression than traditional screws due to the additive property of each thread providing compression. Mechanical studies show that the Acutrak screw provides the greatest push-out force, highest amount of retained compression after cyclic loading, and highest resistance to torsional loading compared to AO and Herbert screws in cadaveric and synthetic bone material.2
Backed by more than 25 years of clinical data and referenced in more than 100 studies in peer-reviewed journals, the Acutrak family of screws has demonstrated efficacy in hand, wrist, foot, and ankle applications. More than 1.5 million Acutrak® and Acutrak 2® screws have been implanted to date.3
For more information about the Acutrak product line, please visit http://www.acumed.net/products/screw-pin.
###################################
About Acumed
Acumed LLC is a global leader of innovative orthopaedic implant solutions. Founded in 1988, Acumed is headquartered in Hillsboro, Oregon, with offices and a distribution network around the world. Acumed is dedicated to developing products, service methods, and approaches that improve patient care.
Availability
These materials contain information about products that may or may not be available in any particular country or may be available under different trademarks in different countries. The products may be approved or cleared by governmental regulatory organizations for sale or use with different indications or restrictions in different countries. Products may not be approved for use in all countries. Nothing contained on these materials should be construed as a promotion or solicitation for any product or for the use of any product in a particular way which is not authorized under the laws and regulations of the country where the reader is located. Specific questions physicians may have about the availability and use of the products described on these materials should be directed to their particular local sales representative. Specific questions patients may have about the use of the products described in these materials or the appropriateness for their own conditions should be directed to their own physician.
References
1. Data on file at Acumed
2. Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res.1998;350:237–245.
3. Acumed sales reports