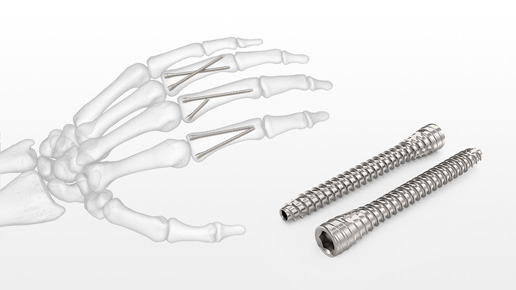
NanoPhix™ Cannulated Lag Screw
For treatment of Hand Fracture Management

Overview
The NanoPhix cannulated lag screw system features 1.5 mm diameter implants and an innovative dual diameter guide wire that simplifies implant placement.
Related Documents
Also related to Hand Fracture Management procedure:
Acutrak 3 Headless Compression Screw System
Acutrak 3 is the latest innovation in our headless compression screw technology and is comprised of 93 unique screws that include the introduction of a new 2.0 mm diameter Nano screw and extensions to...
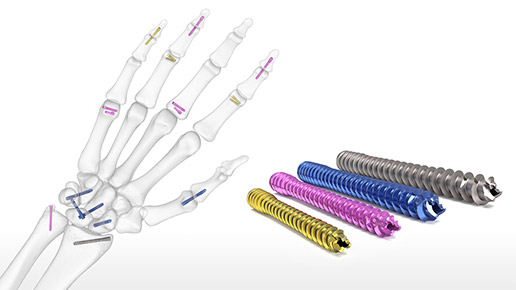
Hand Fracture System
The Acumed Hand Fracture System is designed to surgically treat various metacarpal and phalangeal fractures, fusions, and osteotomies and includes five solutions in one tray....
InFrame Intramedullary Threaded Micro Nail
The InFrame Intramedullary Threaded Micro Nail system features a 2.0 mm diameter, stainless steel micro nail with a non-compressive design to achieve various implantation constructs for phalangeal fra...
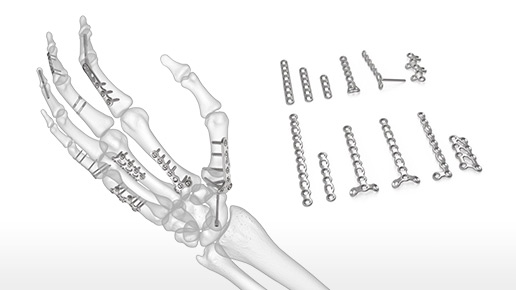
INnate Intramedullary Threaded Nail
The INnate Intramedullary Threaded Nail system features a dual diameter, stainless steel nail with a noncompressive construct to achieve canal fill and stable fixation of metacarpal fractures, avoidin...

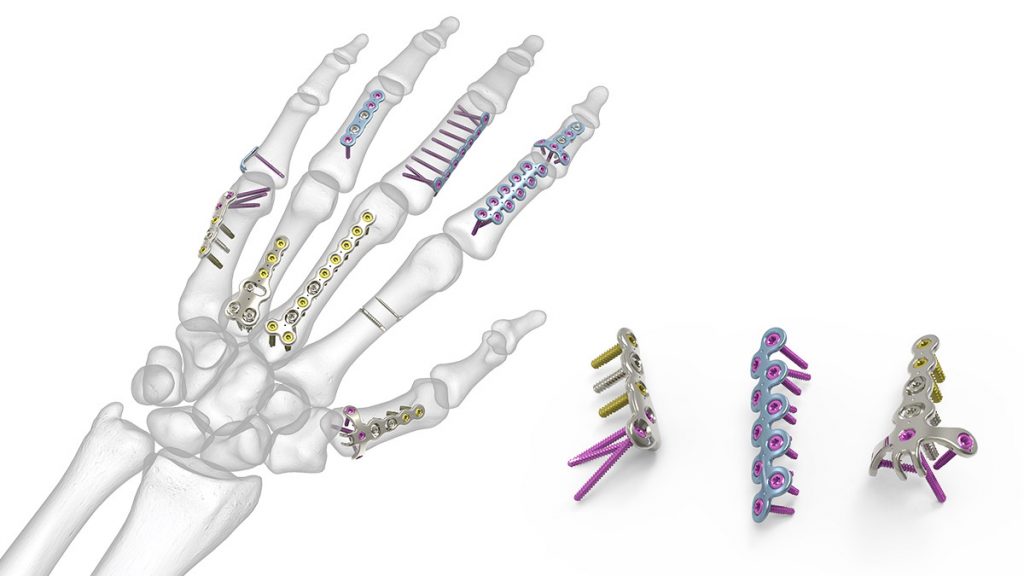
OsteoMed HPS Hand Plating System
The OsteoMed Hand Plating System (HPS) features low-profile implants and instrumentation designed specifically for treating hand trauma. The HPS Plates are available in four size modules: 1.2, 1.6, 2....
Comprehensive System
The system consists of 21 implants from 6–26 mm in 1 mm increments, 2 dual-diameter guide wires, a depth gauge with a countersink tip, and a 1/16” hex driver. Implants and instruments come sterile packed.
NanoPhix major and minor diameter: 1.5 mm/1.2 mm
NanoPhix head diameter: 2.5 mm
Guide wire length and diameters: (trailing) 75 mm length x .6 mm dia. and (leading) 106 mm length x 1.3 mm dia.

System Features
Depth gauge features a countersink tip to minimize screw head prominence.


Specifically sized for small fracture fragments to facilitate early, active mobilization for accelerated healing and faster return to daily activities.
Cannulated, 1.5 mm diameter design allows simple and precise fixation of small fracture fragments such as avulsion and condylar fractures.
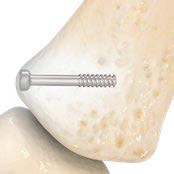

Innovative dual-diameter guide wire eliminates the need for drilling, simplifying a more precise implant placement.
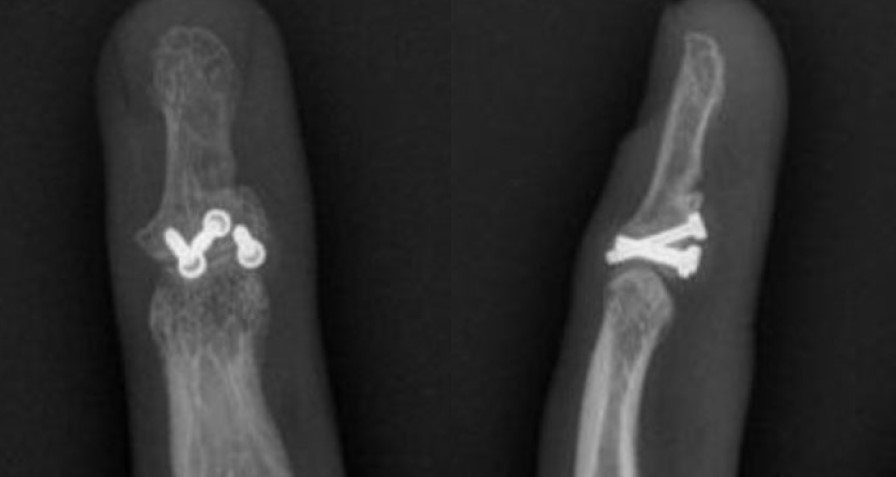
X-Rays
Current Procedure Terminology (CPT) Codes
Code : Procedure
- 26720 : Closed treatment of phalangeal shaft fracture, proximal or middle phalanx, finger or thumb; without manipulation, each
- 26725 : Closed treatment of phalangeal shaft fracture, proximal or middle phalanx, finger or thumb; with manipulation, with or without skin or skeletal traction, each
- 26727 : Percutaneous skeletal fixation of unstable phalangeal shaft fracture, proximal or middle phalanx, finger or thumb, with manipulation, each
- 26735 : Open treatment of phalangeal shaft fracture, proximal or middle phalanx, finger or thumb, includes internal fixation, when performed, each
- 26750 : Closed treatment of distal phalangeal fracture, finger or thumb; without manipulation, each
- 26755 : Closed treatment of distal phalangeal fracture, finger or thumb; with manipulation, each
- 26756 : Percutaneous skeletal fixation of distal phalangeal fracture, finger or thumb, each
- 26765 : Open treatment of distal phalangeal fracture, finger or thumb, includes internal fixation, when performed, each
References
ExsoMed™ Corporation is a wholly owned subsidiary of Acumed LLC.
Acumed® and NanoPhix™ are registered trademarks of Acumed LLC.