
Manufacturer
Indicates the medical device manufacturer, as defined by EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. (reference 5.1.1*)

Date of Manufacture
Indicates the date when the medical device was manufactured. (reference 5.1.3*)
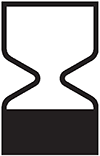
Use-by date
Indicates the date after which the medical device is not to be used. (reference 5.1.4*)

Authorized representative in the European Community
Indicates the authorized representative in the European Community. (reference 5.1.2*)

Batch Code
Indicates the manufacturer’s batch code so that the batch or lot can be identified. (reference 5.1.5*)

Catalogue number
Indicates the manufacturer’s catalogue number so that the medical device can be identified. (reference 5.1.6*)

Sterilized using ethylene oxide
Indicates a medical device that has been sterilized using ethylene oxide. (reference 5.2.3*)

Sterilized using irradiation
Indicates a medical device that has been sterilized using irradiation. (reference 5.2.4*)

Do not resterilize
Indicates a medical device that is not to be resterilized. (reference 5.2.6*)

Non-sterile
Indicates a medical device that has not been subjected to a sterilization process. (reference 5.2.7*)

Do not use if package is damaged
Indicates a medical device that should not be used if the package has been damaged or opened. (reference 5.2.8*)
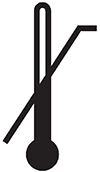
Upper limit of temperature
Indicates the upper limit of temperature to which the medical device can be safely exposed. (reference 5.3.6*)
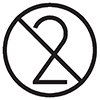
Do not re-use
Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. (reference 5.4.2*)

Consult instructions for use
Indicates the need for the user to consult the instructions for use. (reference 5.4.3 *, **)

Caution
Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself. (reference 5.4.4*)

MR Safe
An item that poses no known hazards resulting from exposure to any MR environment. MR Safe items are composed of materials that are electrically nonconductive, nonmetallic, and nonmagnetic. (7.3.1***)

MR Conditional
An item with demonstrated safety in the MR environment within defined conditions. (7.3.2***)

MR Unsafe
An item which poses unacceptable risks to the patient, medical staff or other persons within the MR environment. (7.3.3***)
* ISO 15223-1 2nd Ed. 2012-07-01, Medical Devices - Symbols to be used with medical devices labels, labeling, and information to be supplied - Part 1: General requirements
** An eIFU indicator below symbol 5.4.3 indicates the website URL where instructions for use are made available in electronic format.
*** ASTM F2503 International Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment